Chemical Synthesis
At ISurTec, we have a fully functional wet lab for small molecule and polymer synthesis. We design our own synthetic routes and perform reactions on a bench scale. We have in-house GPC, FTIR, and UV/fluorescence spectrophotometry for characterization, as well as access to the nearby University of Minnesota facilities. We specialize in photoreactive small molecules and polymers.
Photochemistry
Photochemistry uses light to cause chemical reactions. All things absorb different wavelengths of light, giving them their color. The energy in that light can also be used to break and reform chemical bonds if the light and the chemical are chosen correctly. An example of this type of reaction is a photoinitiator. After absorbing light, a chemical bond can break homolytically to form two radicals. The radicals can react with monomers to polymerize or they can react with another radical to reform covalent bonds.
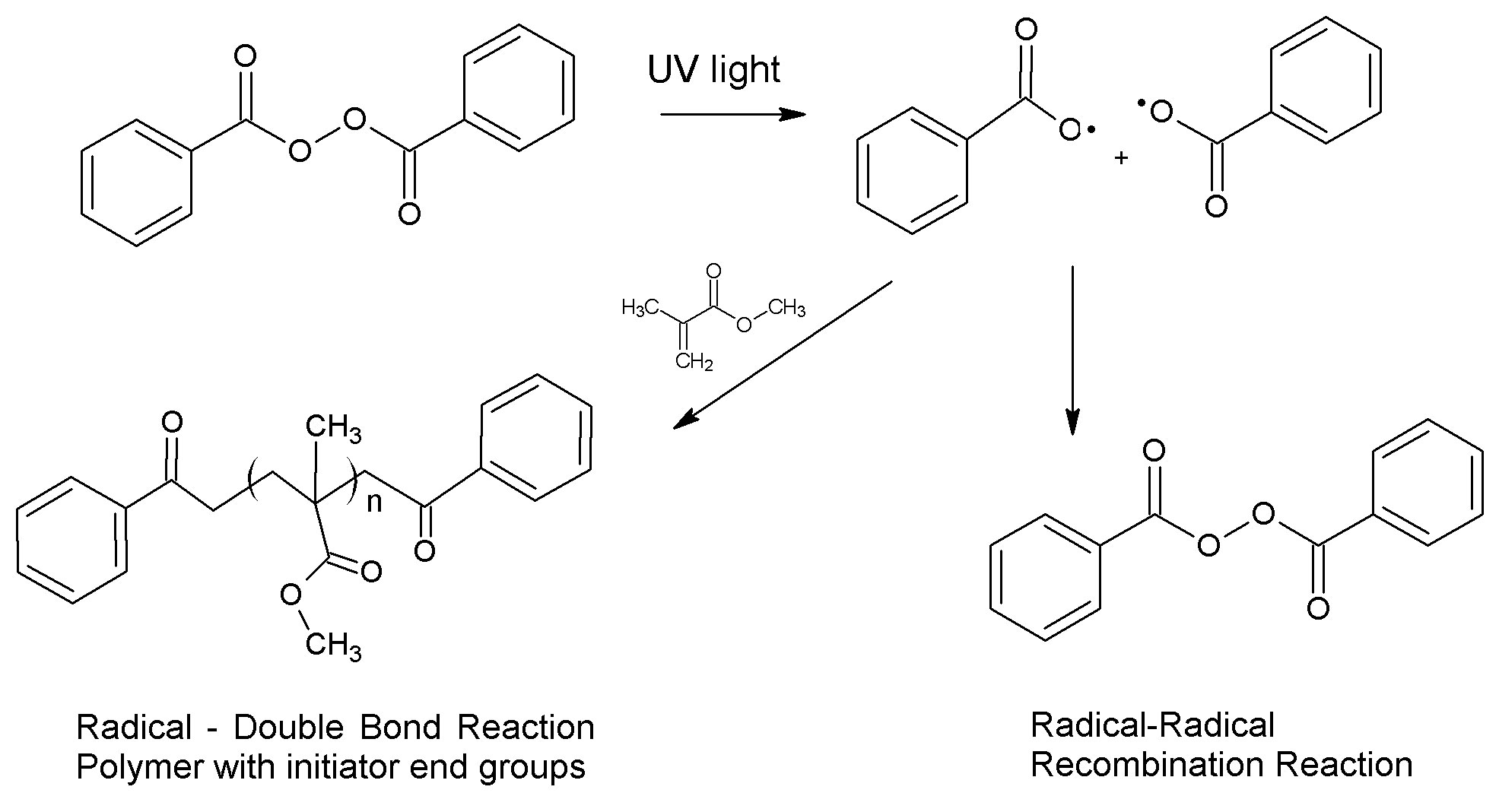
Benzoyl peroxide photoinitiation of methylmethacrylate polymerization
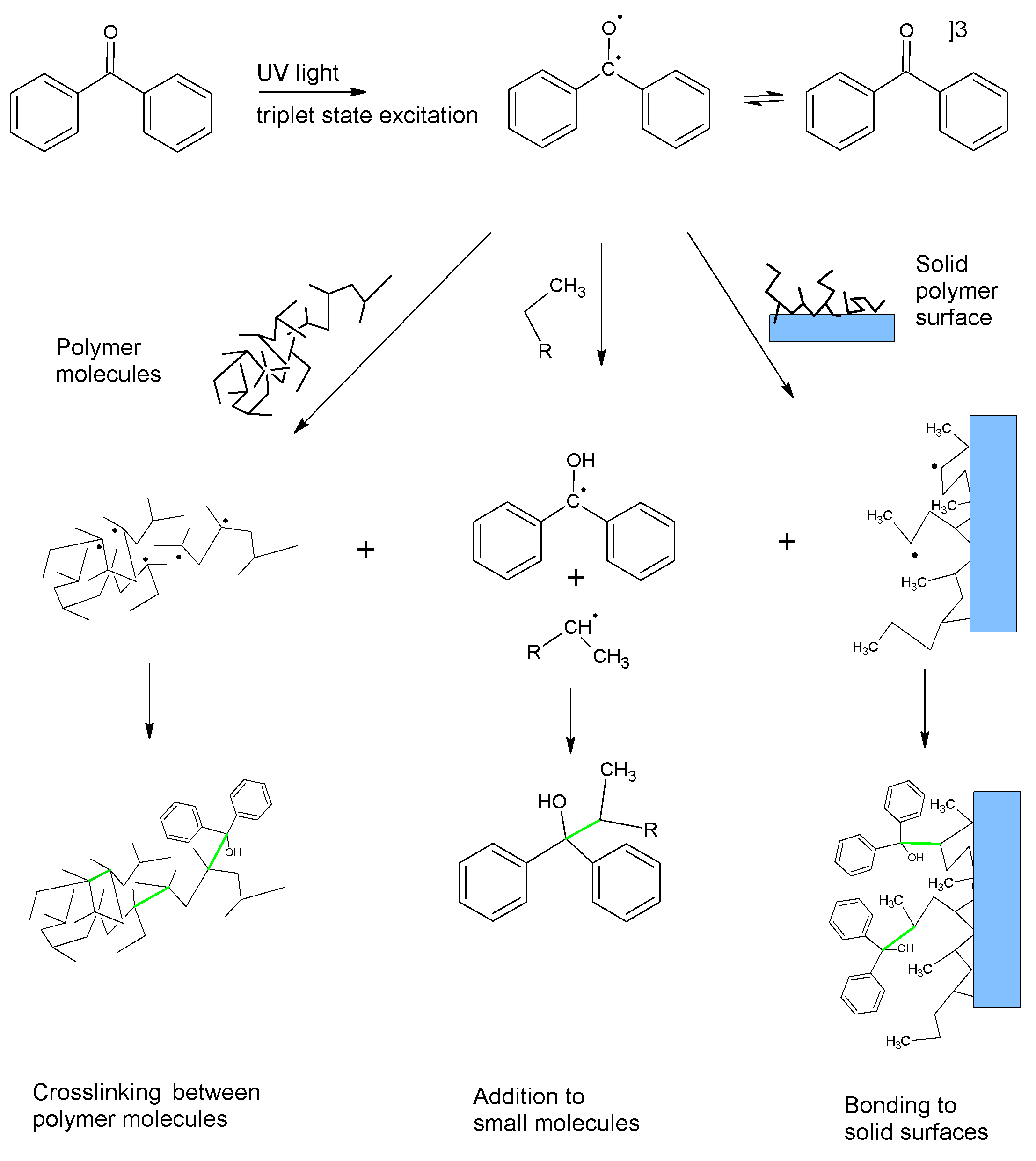
Similarly, other photoreactive molecules can absorb light to enter an excited state in which they act as pseudo-diradicals. In this state the molecules can abstract hydrogen atoms, creating radicals on otherwise unreactive molecules. If the radicals are on a polymer or polymeric substrate, they can recombine, forming covalent bonds in a process called crosslinking. These new carbon-carbon bonds are strong and provide durability to the crosslinked material.

ISurTec is a technology innovator with a deep passion for identifying, creating and commercializing new methods and products that enable companies to fulfill their mission.
CONTACT
Innovative Surface Technologies, Inc.
1045 Westgate Drive Suite 100
Saint Paul, MN 55114
651-209-9757